INTRODUCTION
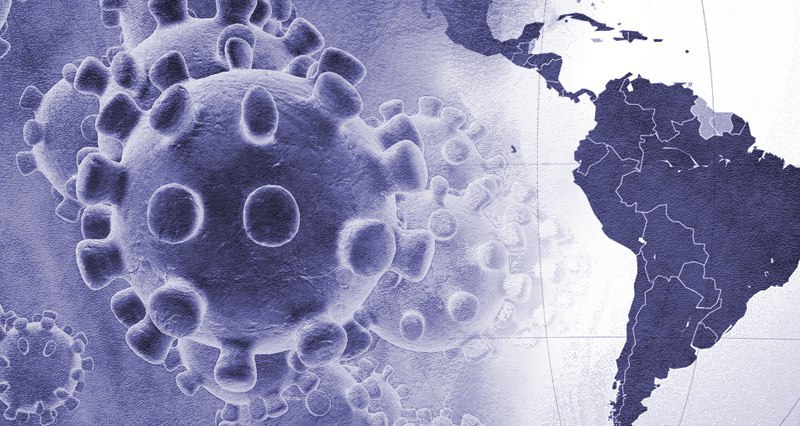
The pandemic derived from the Covid_19 has attacked almost everyone to a different extent. The Ibero-American region has been one of those significantly affected. While the first cases of the virus came later than elsewhere such as Europe or the United States, the truth is that in some Latin American countries its impact has been much greater, with more dead relative to the number of infected.
The health systems has been saturated around the world, but iberoamerica has especially seen its inability to assist the infected.
Ibero-America as a territory is one of the richest places in the world in relation to the amount of resources it has and the small population it inhabits in relation to the space it encompasses. For this reason it is targeted by different powers and where the management of the pandemic has a view, too, from a geopolitical aspect.
The first country to announce that it would have the vaccine ready was Russia, and immediately other powers reacted by saying the same thing, as if it were clear competition. Powers such as China, Russia, the United Kingdom, the United States and private multinational companies have been trying to reach trade agreements to provide the vaccine against Covid_19 to Latin American countries.
Today more than 100 vaccines to respond to the Covid_19 are developing on the planet. There are more than 26 million infected and the number of deaths is approaching one million inhabitants, where Ibero-America is sadly one of the largest epicentres of contagion.
PROVIDERS
China
China has four anti COVID-19 vaccines in the final phase of clinical trials. At least three of them have already been offered to essential workers under an emergency use program started in July. Today the Internet has been flooded with photos and videos showing the return to normalcy throughout China, including the epicenter of Wuhan.
China is one of the main countries interested in Latin America, which is why it has carried out intense cooperation with different countries and supranational organizations. In particular, the Pan American Health Organization has highlighted the cooperation between China and Latin America to obtain a vaccine against Covid-19 as soon as possible. In the same Pan-American organization, it was highlighted before a Xinhua consultation that they are carrying out efforts and collaboration with countries such as Argentina, Peru, Mexico and Brazil for the development of vaccines with China. From this international organization, the cooperation throughout the continent that is being carried out among the Ibero-American countries is exalted.
According to the Pan American Health Organization, Argentina and Brazil play a key role in producing and distributing vaccines due to their trajectory in this area and their distribution networks. China, despite being “the world’s factory” cannot produce vaccines for everyone, hence it is interested in negotiating with the most optimal countries in the regions next to vaccinate.
From Latin America these agreements with China are being applauded, considering it a public good destined for the health of the entire region. But the matter goes further, the Chinese leadership has moved its influences and today is presented as the main commercial partner of many of the Latin American countries.
Brazil was already producing “Coronavac”, a Chinese-origin drug against COVID-19, and now it is expected that before the end of the year another state vaccine factory will be inaugurated in Sao Paulo (the most industrial and populated area of Brazil).
For the creation of the new factory, large donations are expected to finance the project in order to increase the production of vaccines of the Butantan Institute against COVID-19. For this, the new vaccine manufacturing plant finds the financial support of Brazilian companies and other multinationals based in Brazil, through the fund against the coronavirus. At the moment, just over 17 million dollars have been raised.
At the moment the Chinese vaccine is being tested in 9,000 Brazilian volunteers. According to estimates by the Brazilian authorities, vaccinations in the country are expected to begin in December. They are estimated to be able to produce around 60 million doses between December and March if they get authorization from the National Health Surveillance Agency.
On the other hand, in the case of Argentina, since the end of August they began with phase III of the clinical trials of the vaccine developed by China. The Argentine Minister of Health, Ginés Gonzáles García expressed his joy at the advances of the Chinese vaccine. This Argentine-Chinese agreement was made in conjunction with the National Pharmaceutical Group of China (Sinopharm Group) belonging to China National Biotec, and the gigantic Argentine laboratory Elea Phoenix belonging to the powerful Gold, Sigman and Sielecki families. Argentina’s connections with China with these agreements seek from a geopolitical point of view to further strengthen relations, especially in the face of a disastrous management by Alberto Fernández that is weakening the government.
In the Peruvian case, in August CNBG and Sinopharm Group were authorized to carry out third-phase clinical trials together with experts from the Cayetano Heredia University and the San Marcos National University.
Finally, Mexico is another target in the crosshairs. Also in August, it agreed with the Chinese companies Cansino Biologics and Walvax Biotechnology that it will also participate in phase 3 of the clinical trial of the vaccine.
Phase 3 of clinical trials typically involves thousands of people to verify the safety and effectiveness of the vaccines that are key to their approval on the market. China has argued that it is necessary to carry out clinical trials in other parts because its population is not in a condition to be subjected to trials due to having the epidemic under control.
According to a guideline for the clinical evaluation of COVID-19 vaccines recently published by the National Medical Products Administration, the vaccine should offer immunity for at least six months and preferably for more than one year.
USA
The United States is the country most affected by the pandemic worldwide, according to official statistics, more than 6 million infected have been recorded, more than 30,000 every 24 hours, with a balance of almost 200,000 deaths.
The United States has been trying to develop a vaccine against Covid-19 for some time, in fact it is one of the countries that searched for a vaccine shortly before the WHO declared the pandemic. Despite this, they have not yet found the vaccine.
Donald Trump recently expressed that perhaps the vaccine could be ready by the end of October, erroneous information as clarified by the Director of the Centers for Disease Control and Prevention, Robert Redfield, who assured that the American president was wrong. On the other hand, a group of experts in infectious diseases expressed concern that the US government is putting pressure on the Food and Drug Administration to approve the vaccine before carrying out all the necessary tests and safety control. Particularly Redifield assured that “at the earliest” the first samples of the vaccines could arrive in the months of November or December, but under no circumstances form part of a vaccine ready to be incubated in the entire American population. Redfield believes that the vaccine could be ready for the entire population after a period between 6 and 9 months, that is, during the third quarter of 2021.
There has been no information about whether there are plans to sell the vaccine to Latin American countries, but we well know that Donald Trump months ago declared that he wanted the vaccine exclusively for his people.
On the other hand, and from a private point of view, there is the initiative of the multinational company Johnson & Johnson that has been working over the last few months to try to develop a vaccine against Covid-19. The multimillion-dollar company was conducting tests in Belgium, the United States and other countries such as Germany, Spain and the Netherlands. They recently announced that they are in an advanced stage of the vaccine so they were looking for 20,000 volunteers from Latin America to include this region in the market target. Josue Bacaltchuk is the vice president of Johnson & Johnson’s Belta division, she said that the countries that are testing their vaccines would probably be the first to have access to their vaccine.
A clear objective of this multinational company is to be able to operate in Brazil, since it is the largest and most affected country, although they have also shown interest in other countries such as Colombia and Argentina, also being the largest in South America after Brazil. But the desire to reach more Ibero-American countries has led Johnson & Johnson to extend initial plans to include Peru, Mexico on its list, while it is in talks to expand further in the Ibero-American region.
UK
The United Kingdom announced in April that it could have the Coronavirus vaccine ready by August. However, in August, the vaccine situation was still very immature. From the University of Oxford, Sarah Gilbert indicated that in September it was very likely that the vaccine could be ready to start being used at the European level. But already at the end of the month of September, we did not see evidence of it either, on the contrary, it has suffered numerous.
The British bet comes at the hands of AstraZeneca, a multinational pharmaceutical company that has long operated in more than 100 countries. This company is positioned as the fifth largest pharmaceutical company in the world, and a major leading company in the United Kingdom that is listed on the London and New York Stock Exchanges.
AstraZeneca’s offensive was very large, worldwide, but it has shown special interest in the Ibero-American region, managing to reach an agreement with numerous regional countries. Mainly their bet goes to Argentina and Mexico, the largest Spanish-speaking countries, but they have also been conducting individually with other countries in the region. However, the deal with Argentina and Mexico is due to the industrial facilities that these countries have in laboratories, having ruled out the possibility of Brazil, which is also well prepared for this task. (Maybe it is because of the Russian and Chinese advance in the agreements).
The fact is that the Mexican billionaire Carlos Slim’s foundation is an intermediary in the agreement to produce the vaccine. This measure was applauded by the Community of Latin American and Caribbean States (CELAC).
AstraZeneca would be developing the vaccine in conjunction with the University of Oxford, with the collaboration of laboratories from Mexico and Argentina, where curiously in the Argentine case, AstraZeneca chose mAbxcience. Mabxcience is a laboratory created just a few months ago, more specifically in February, it is owned by Hugo Sigman, a billionaire owner of magazines and other medical companies. Sigman is a wealthy left-wing political man who was implicated in the ephedrine mafia, the 500% increase in alcohol in Gel shortly before the pandemic, and even long ago, being linked to the communist guerrilla ERP.
In any case, with the approval of CELAC, many Latin American countries were in favor of the agreements reached by Argentina and Mexico with the British pharmaceutical company. The drugmaker’s goals were to be able to mass-produce the vaccine from the first quarter of 2021. But recent trial failures with negative effects on some of the volunteers appear to be likely to delay this further. This vaccine, which is expected to produce 250 million doses, would be distributed by Argentina and Mexico at cost in Latin America, except Brazil, which has a separate agreement.
Russia
The Russian vaccine Sputnik-V (V for vaccine), was the first to be registered in the world in August this year. The development is carried out by the Nikolay Gamaleya Center for Epidemeology and Microbiology. The news of the Russian vaccine was the scene of attacks and misrepresentations that criticized its reliability, however, in September it was the first published in the academic journal The Lancet, showing stable immunogenicity. The tests were carried out on more than 40,000 Russian volunteers, giving immediate results a significant increase in antibodies to Covid_19, even more than those who had recovered after being infected.
Russia was also very interested in entering Latin America as a potential supplier of the vaccine against Covid_19. Russia is particularly interested not only in the sale of the vaccine, but also as a platform for clinical analysis and joint production. The Russian forecast is to provide 130 million vaccines to the region, of which 32 million would be destined for Mexico, as announced by the Russian Fund for Direct Investment. Kiril Dmítriev, director of the fund, stressed that about 25% of the Mexican population will be able to access the Russian Sputnik V vaccine in an effective and safe way. The first deliveries would be arriving this November.
According to the Russian Fund for Direct Investment, most Latin American countries have contacted Moscow in order to begin negotiations to acquire the Sputnik V vaccine.
On the other hand, another important agreement was carried out by Russia with Brazil, where the supply of 50 million vaccines for the state of Bahia has been agreed for the moment. Other states of Brazil, such as Paraná, will take another rhythm where it is expected that clinical trials will be carried out by the beginning of 2021.
Another beneficiary country would be Venezuela, which is already initiating procedures to participate in phase 3 of Sputnik V clinical trials. The possibility of producing the vaccine in Nicaragua is also being studied.
Conclusions
States and laboratories have seen in Latin America a special place for which to compete. It is an underdeveloped area in many ways that is suffering from a sorry state of the pandemic. The shortcomings of these territories and the number of cases that exist make up an ideal field for those who want to sell vaccines. The interests to introduce this vaccine go through the strategic, political and even economic, and for this reason Latin America is usually in the focus of permanent interest of the great powers.
The Chinese case has shown particularly great silence regarding its vaccines, at least the one that is being tested in Latin America of which it is not known if it is part of those that have been tested in the Asian country, or if Latin America would be a “testing ground” dedicated to him.
Another striking aspect that we can highlight are the agreements with the British pharmaceutical company and Oxford with Argentina and Mexico, when, curiously, left-wing governments are negotiating with the British multinational, making more propaganda and showing more interest in it than in alternatives such as those of other countries like Russia. The opposite case has been demonstrated with Brazil, the country that has most promoted agreements with Russia for the production and distribution of the Sputnik V vaccine.














Leave a Reply